Dr Emma Ravichandran & Dr Paula Mann recently wrote an article for the Journal of Aesthetic Nursing on different filler techniques for the lips. This article is aimed mainly at medical aesthetic practitioners but we hope that you find it interesting if you read to the end.
Filler Techniques for Rejuvenating, Revitalising and Restoring
Lip filling with hyaluronic acid dermal filller is not a recent phenomenon. In 2017, lip augmentation was the most popular non-surgical cosmetic treatment.
In regard to celebrity cultrue, figures like Angelina Jolie originally caught the public interest towards the naturally plump lip in 2000, but it was years later that other public figures initiated the growing popularity of with lip augmentation. The American Society of Plastic Surgeons reported an increas of 50% in lip augmentation for 18-55 year olds between 2000 – 2016.
In the UK, images of public figures before and after their lip augmenation are readily available. Here at Clinetix, a 15% annual increase in requests for lip treatments has been documented over the past four years. Recently, there have been significant advances in dermal filler technology, and several new lip products are available in the UK. Practitioners now have a better understanding of filler rheology and the vivo biointegration of products (Sundaram et al, 2015; Tran et al, 2014). This information, coupled with an in-depth understanding of lip anatomy, affords practitioners the tools to naturally and effectively rejuvenate and augment lips in artistic fashion.
Anatomy of the lips
The upper and lower lip are made up of three different tissue zones; the white lip, the vermillion border and the vermillion. The white labial zone (white lip) is skin with stratified squamous epithelium, dermis sweat glands and hair follicles overlying the subcutaneous tissues. The vermillion border is a unique tissue which is a transition zone between the white and red lip. It is stratified squamous epithelium with no melanocytes present, dermis with no sweat glands or hair follicles overlying the subcutaneous tissues. The vermillion (red lip) is specialised mucosa, lamina propria with minor salivary glands overlying the submucosal tissues.
In the cross section, from external to internal, the lips are skin, subcutaneous fat, the circumferential muscle obicularis, submucosal fat and oral mucosa. The free end of the lips are specialised dry mucosa. The blood supply to the lip is from the facial artery via the upper and lower labial arteries and there is a variance in the depth and anatomy of the labial arteries. The main artery will lie intramuscularly with smaller branches becoming more superficial to supply overlying tissues.
If all these tissues zones are histologically different, it stands to reason that they will display different changes with ageing that will culminate in the physical signs of ageing seen around the lip area. Therefore, it also stands to reason that these tissues should be treated individually when performing true lip rejuvenation for patients.
The Ageing Process
Lips are particularly vunerable to showing signs of ageing, becoming thinner and wider, there is a reduction of anterior tooth show. Furthermore, the philtrum columns and vermillion border lose strength and definition, the philtrum and cupid’s bow become flattened and perioral lines develop. The nasolabial folds also become more obvious (Figure 1).

The ageing process is multifactorial and includes skeletal, dental, skin, fat and muscle changes.
With age, the maxilla shows generalised resorption with an increase in the maxillary angle, and the pyriform aperture increases in size. The body of the mandible and ramus will reduce in height, while the alveolar bone shows gradual resorption which is significantly accelerated by loss of teeth. The loss of support from skeletal base and teeth leads to a lack of anterior projection and descent of all the soft tissues.
Chronological tooth wear or the event of tooth loss will reduce the occlusal vertical dimension and diminish the support to the lips.
The deep and superficial fat compartments and planes become lipodystrophic, resulting in wrinkling of the skin and descent of overlying soft tissues.
Aged skin shows epidermal thinning, with flatenning of the dermal-epidermal junction. There is loss of tensile strength and decreased elasticity of the skin. Ageing skin also produces less oil, which can lead to chronic dryness.
Loss of skin elasticity, subcutaneous volume and bony support, in addition to repeated perioral muscle activitiy, contributes to perioral rhytides. These can be made worse by environmental factors, such as excessive sun exposure or smoking.
Augmentation and rejuvenation: the approaches to lip treatment
Lip augmentation can be performed with many different techniques and with many different products. Results can range from attractive, natural, balnaced and in harmony with the face to disproportionate, unnatural and detrimental to the overall aesthetic apearance of a person.
In the authors’ experience, careful assessment, diagnosis, treatment planning with appropriate fillers and good technique will consistently deliver excellent natural rejuvenation with or without augmentation. Adequate training, studying and experience are essential before performing these treatments for patients.
Traditionally, lip treatments have been carried out using one hyaluronic acid product and one technique. A detailed understanding of the anatomy of the different tissues comprising the lip complex and the individual nature of the ageing process of each tissue reflects the need for a multimodal approach to both technique and product choce for true rejuvenation or augmentation of the lips. This is referred to as the combination approach.
Combination Approaches
Lip Augmentation
The purpose of lip augmentation treatment is to enlarge and define the borderes of the lips for a more aesthetically desirable outcome. Traditionally, one hyaluronic acid (HA) product has served both of these requirements. The superficial fat compartment of the white lip, vermillion border and red lip are continours and there is potential for migtation of HA under these three tissues. For this reason, an elastic, cohesive product (BELOTERO® Lips Shape) should be placed submucosally in the vermillion (body) of the lip only.
To define the vermillion border, a second cohesive, low viscosity product (BELOTERO® Lips Contour) should be used and injected intradermally. Intradermal placement avoids potential migration of HA from the vermillion border into the white lip, which would adversely round the vermillion border and change the profile and tecture of the white lip. The cohesive polydensified matrix (CPM®) technology of both BELOTERO® Lips Contour and Shape lift in areas that require lifting and filling and will blend and integrate where no lifting and filling is required.
For a practitioner new to lip augmentation, generally, it is easier to start with lips are relatively youthful and simply require to be slightly enlarged and defined.
Lip Rejuvenation
Lip rejuvenation aims to restore a youthful appearance to the lips. Procedures will take into account the individual ageing processes affecting a patient’s lip and perioral area and involves multiple treatment techniques to address multiple issues. Lip rejuvenation requires restoration of strength and structure to the vermillion border, philtrum columns and to replace subcutaneous/ submucosal volume depletion. Lip rejuvenation requires at least two products with different rhelological properties to address the different tissue types in the lips. An elastic and cohesive product (BELOTERO® Lips Shape) is needed to replace lost volume in the vermillion and, potentially, in the mesolabial zone, while a cohesive low-viscosity product (BELOTERO® Lips Contour) should be used to restore structure of the vermillion border, white lip and philtrum columns. BELOTERO® Lips Contour can be injected intradermally without causing tissue Tyndalling, and this intradermal locking of the product minimises the potential for unwanted migration.
Techniques: Definition and Volumisation
There are two techniques to learn for placement of dermal fillers in the lips: definition of the vermillion border (the junction between the red lip and the skin) and volumisation of the vermillion (the red part of the lip).
Lip Definition
To improve the definition or sharpness of the vermillion border, a cohesive, low-viscosity filler is require; BELOTERO® Lips Contour is perfect for this indication (Figure 2). Instead of injecting underneath the dermis as for volume replacement, the goal is to place the filler within the dermis in straight lines along the vermilion border. For this reason, a (typically 30g) narrow-gauge needle is used, as a cannula will preferentially slide into the subdermal or deepeer tissue planes.
Prior to treatment, the area is always thoroughly cleaned and disinfected with a skin disinfectant, such as a chlorhexidine solution or Clinisept.
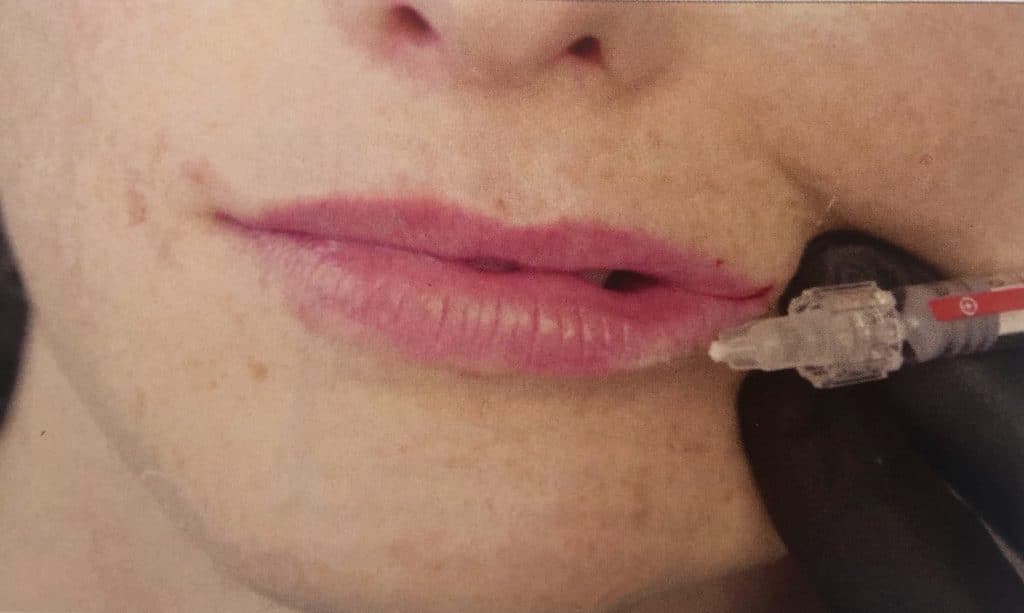
Lip Volumisation with a cannula
The goal in volumisation is to introduce a dermal filler into the vermillion of the lip, just underneath the mucosa in the submucosal fat plane. Injecting filler with a needle is possible; however, the advent of cannula has allowed this placement to be performed with potentially less injections, less swelling and bruising for the patient.
A 25g 38mm cannula can be used for this procedure. Volumisation requires a dermal filler that is lifting, elastic and cohesive. BELOTERO® Lips Shape is an ideal choice (Figure 3).
Prior to treatment the area is always thoroughly cleaned and disinfected with a skin disinfectant such as chlorhexidine solution or Clinisept.
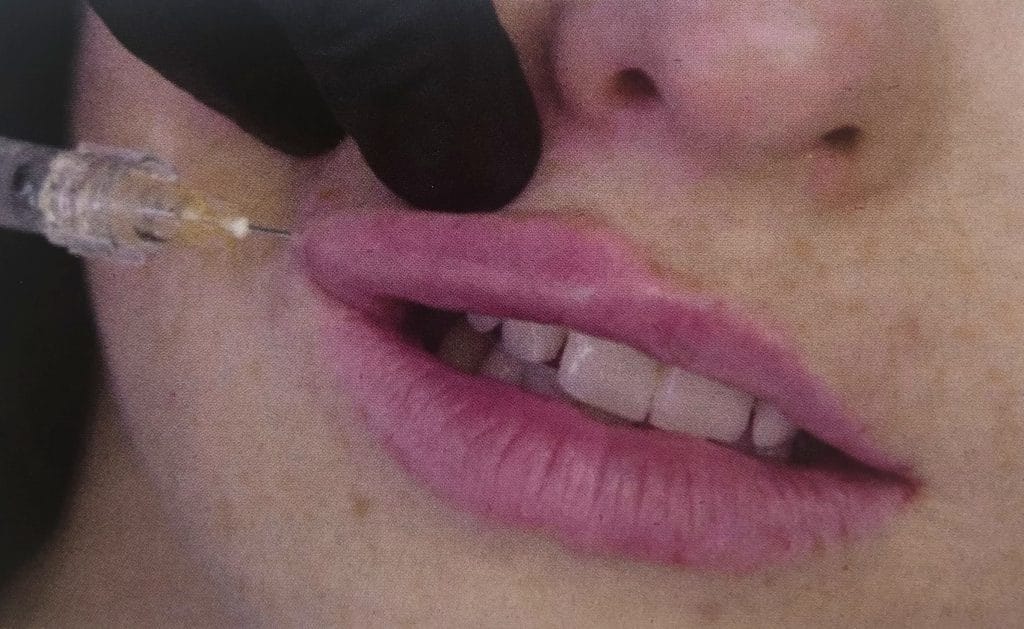
Volumisation with a needle
A 27g 0.5 inch needle can be used for this procedure. Volumisation requires a dermal filler that is lifting, elastic and cohesive. BELOTERO® Lips Shape is an ideal choice.
Prior to treatment, the area is always thoroughly cleaned and disinfected with a disinfectant such as Chlorhexidine solution or Clinisept.
True mastery of these procedures requires understanding of the anatomy and the behaviour of the dermal filler implants in the tissue, as well as a great deal of practice and reviewing of results.

Hyaluronic acid fillers
By definition, HA is a glycosaminoglycan which consists of regular repeating nonsulfated disaccharide units of glucuronic acid and N-acetylglucosamine (Buntrock et al, 2013). This natural biopolymer echibits no species and no tissue specificity (Alberts et al, 2002). It is biodegradable and does not elicit an immune response (Gold, 2007). HA occurs naturally in the body’s cells and tissue fluids, and is a key molecule involved in maintaining the skin’s moisture.
HA Dermal fillers consitist of long chains of HA that have been cross-linked with a chemical, such as 1.4-butanedioldiglycidyl ether (BDDE). Cross-linking stabilises the product to resist rapid degradation by natural hyalase. The HA that is used as a dermal filler is extracted from rooster combs or, more commonly, made by bacteria in the laboratory.
HA dermal fillers are gels manufactured to be injected into or below the skin or mucosa replace or augment volume. The moisture retaining capacity of a HA dermal filler makes the skin pliable and plump, and the duration of correction persists until the stabilised gel is completely metabolised (De Boulle et al, 2013). Many HA fillers now available contain a loval anaesthetic agent such as lignocaine, to make the injection procedure comfortable. There are many applications for HA fillers as anti-ageing and beautification treatment modalities, with different properties, such as concentration of HA and degree of cross-linking. Some products are monophasic homogenous gels (e.g. BELOTERO® or Juverderm), while others are biophasic gels (e.g. Restylane). The different product properties result in unique product characteristics of each HA dermal filler, making each suitable for different clinical indications.
BELOTERO® goes through two stages of cross-linking and results in a cohesive polydensified matrix (CPM). The cohesive, low-viscosity profile of BELOTERO® Lip Contour makes it ideal for restoring and defining the vermillion border, while the elastic profile of BELOTERO® Lips Shape means it is a good choice for volumising the body of the lips.
Contraindications of hyaluronic acid dermal fillers
Certain patients should not be treated with hyaluronic acid fillers. These include those:
- Who are below 18 years of age
- Pregnant or breastfeeding
- Who have an infection at the injection site
- Where ther area has had previous permanent dermal filler
- Who have an allergy to any of the components within the product
- Who have bleeding diathesis
Further consideration should be given to those patients whose expectations may not be met by the procedure and who may be body dysmorphic, and caution should be exercised where other medical aesthetic techniques have been carried out.
Common adverse effects of hyaluronic acid dermal fillers
Common adverse effects from HA dermal fillers include:
- Mild discomfort
- Redness
- Swelling
- Bruising (potentiated with antithrombotic medication)
- Nodules/indurations
These common adverse effects are self-limiting. Care in planning and technique can reduce the likelihood of these outcomes. Extremely rare side effects of injection include bacterial infection and herpes reactivation, tissue necrosis, granulomatous foreign body reaction and blindness (Walker et al, 2018).
To make an informed treatment choice and grant full consent, all patients must be made aware of the benefits and risk of proposed treatments.
Research review
The evidence thus far indicates HA dermal fillers are a safe and effective treatment of facial rhytids, folds and volume loss (Fischer et al, 2016). Studies have shown patient experience satisfaction is higher where added local anaesthetic is used (Fischer et al, 2016).
In one randomised single-blind study, 20 subjects were injected in the nasolabial fold with either a cohesive monophasic polydensified filler or a biphasic non-animal stabilised HA filler. Results showed a significant increase in injection satisfaction, with even greater satisfaction where a cohesive polydensified monophasic dermal filler was used (Sundaram et al, 2015 Phillipp-Dormston et al, 2014).
Research for dermal fillers, specifically in the lip area, is less abundant. Evidence is available from a study of 62 subjects using a HA filler in the vermillion border and body of the lips (Allergan Juvederm Volbella). Results show this treatment achieved high patient satisfaction rate of 83%, with an even higher physician satisfaction exoerience of 100% (Ho et al, 2015).
In a further study of 146 patients using HA fillers of different concentrations (BELOTERO® Lips Shape and BELOTERO® Lips Contour), at every evaluation point, more than 93% of patients were very or very much satisfied with the product (Fischer et al, 2016).
In practice, choosing a dermal filler should be based on the depth and location of tissue to be injected, patient expectations, operator experience and knowledge of rheology of the products available.
BELOTERO Lips range of fillers
Merz has created a dual lip dermal filler combination. The syringes have a volume of 0.6ml which is sufficient to create an optimal corrective and aesthetic result.
BELOTERO® Lips Contour
This contains Lignocaine and 22.5mg/ml of hyaluronic acid and is designed to be injected superficially or mid-dermis for lip contour, fine lines or mild oral commisure correction.
BELOTERO® Lips Shape
This dermal filler contains Lignocaine and 25mg/ml of hyaluronic acid and is used to restore or enhance the lip volume and commisure.
The BELOTERO® Lips range can be used individually or in combination to define, project and volumise for a tailored treatment approach. The CPM® technology allows seamless integration with smooth surface contours, while remaining resilient in areas of high muscular activity (Tran et al, 2014).
Conclusion
Dermal fillers are an excellent treatment modality in lip rejuvenation and augmentation. While techniques for the placement of dermal fillers in lips are commonly taught as part of introductory dermal fillers, the authors would argue that it is one of the more complex treatments to master in aesthetic medicine.
To deliver a natural-looking lip rejuvenation or augmentation treatment, the practitioner must have a comprehensive knowledge of the anatomy and the ageing process of the lip region, as well as the science of the dermal fillers they use and techniques that maximise the safety and efficacy of the products.
Without doubt, the ageing of the lip area is a multifactoral process and must be treated in a multimodal fashion. Topical, resurfacing and remodelling treatments, in addition to dermal fillers procedures, should be considered. Dermal filler choice should be based on the depth and location of tissue to be injected, partient expectations and operator experience and knowledge. The BELOTERO® Lips range offers a duo of o.6ml Hyaluronic Acid dermal fillers that address the individual requirements for treatment of the vermillion and the vermillion border. The combination approach offers a gold standard to patients, both young and old. Regarding the placement of the product, technical skill is important to ensure the correct filler is placed at the correct depth for optimum result. The addition of BELOTERO® Lips range to the authors’ practice has improved their ability to individualise and customer lip treatments for all patients.
Case Studies
Case study 1: 29 years old, medically fit female
Previously, the pateint had Botulinum toxin treatments for the upper face, with no history of any dermal filler treatment.
The patient was concerned about the development of early perioral lines and the loss of natural fullness to her lips. She wanted a natural plumping result.
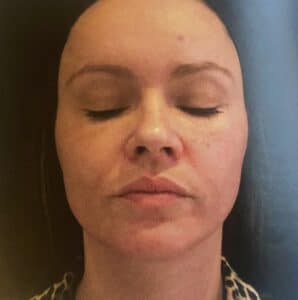

Treatment carried out
The patient had 0.6ml BELOTERO® Lips Shape injected in the submucosal fat of the vermillion using a 25g 1.5ml Cannula (0.2ml upper lip, 0.4ml lower lip), and 0.6ml BELOTERO® Lips Contour injected intradermally in the vermillion border using a 30g 13mm needle (0.3ml ipper lip, 0.3ml lower lip).
2.2ml lignocaine 1:80,000 Adrenaline was used to carry out local anaesthesia for the procedure.
Minimal swelling and no bruising or any other complication was observed.
Photographs were taken immediately before treatment and four weeks after. The patient was delighted with the natural result.
Case Study 2: 81 years old, medically fit female
The patient had not had any other previous aesthetic treatments. She was concerned with lines around the mouth and a lack of lip volume and wished to wear lipstick again to her great granddaughter’s wedding.
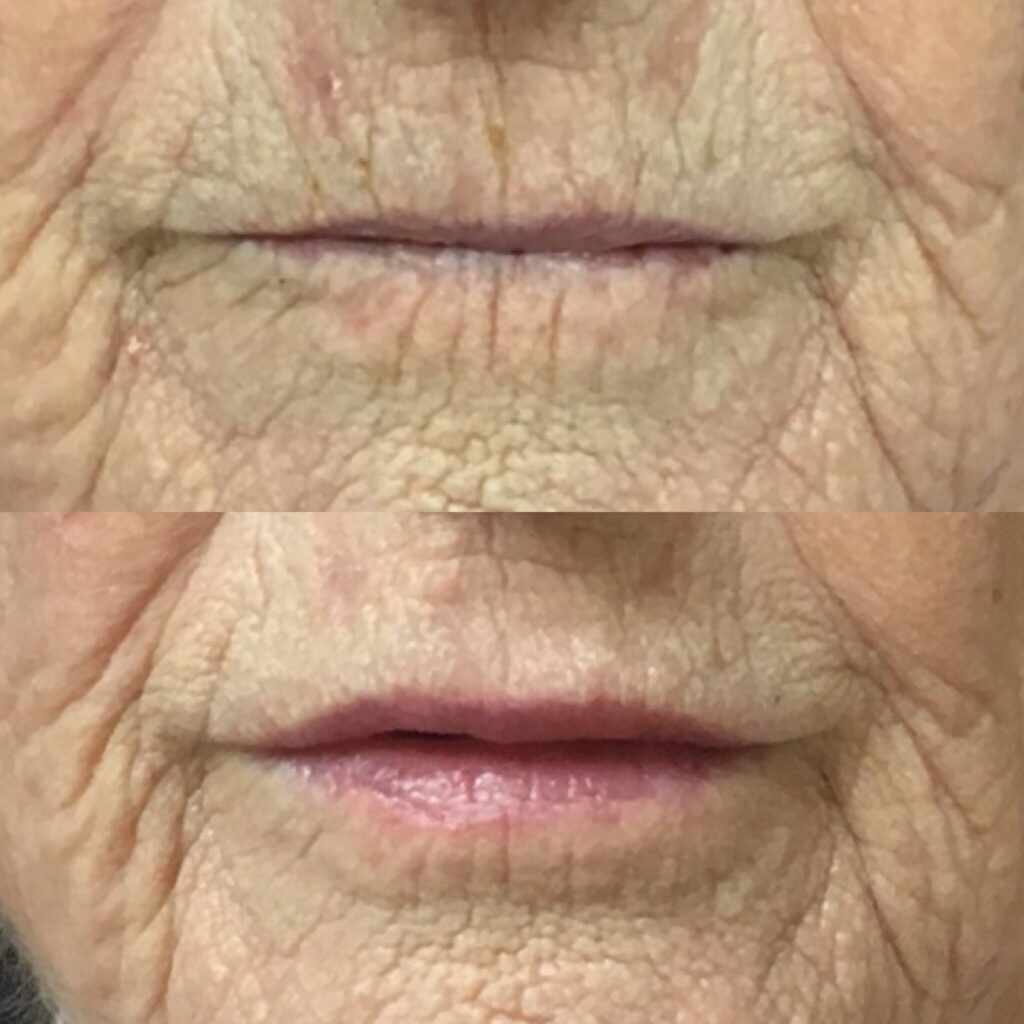
Treatment carried out
The lip area was anaesthetised with 2.2ml Lipgnocaine 1:80,000 adrenaline by intraoral injection.
0.6ml BELOTERO® Lips Shape was injected into the submucosal fat of the vermillion using a 25g 1.5 inch cannula and 0.3ml was placed evenly in the upper and lower lip. Next, 0.6ml of BELOTERO® Lips Contour was placed in the superficial dermis using a blanching technique with multiple 30g 13mm needles.
0.15ml was used in the upper and lower vermillion border and 0.3ml in the perioral lines of the white skin. There was no post operative complications and the patient was very happy with the results.
Photographs show the patient immediately before treatment and four weeks post-treatments.
This article was sponsored by Merz Aesthetics, in association with the Journal of Aesthetic Nursing. The authors of this article are Merz Aesthetics Key Opinion Leaders.
References
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (eds). Molecular biology of the cell, 4th edn. New York (NY): Garland Science, 2002
- Buntrock, H, Tilmann R, Prager W et al. Efficacy, safety and patient satisfaction of a monophasic cohesive and polydensified matrix versus a biphasic nonanimal stabilized hyaluronic acid filler after single injection in nasolabial folds. Dermatol Surg. 2013;39(7):1-9. https://doi.org/10.1111/dsu.12177
- De Boulle K, Glogua R, Kono T et al. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyauronic acid dermal fillers. Dermatol Surg. 2013;39(12):1758-1766. https://doi.org/10.1111/dsu.12301
- Fischer TC, Sattler G, Gauglitz GG. Hyaluronic acid filler containing lidocaine on a CPM basis for lip augmentation: reports from practical experience. Facial Plast Surg. 2015;32(3):283-288. https://doi.org/10.1055/s-0036-1583534
- Gold MH. Use of hyaluronic acid fillers for the treatment of the ageing face. Clin Interv Ageing. 2007;2(3):369-376
- Ho D, Jagedo J. Biological properties of a new volumizing hyaluronic acid filler: a systematic review. J Drugs Dermatol. 2015;14(1):50-54
- Lee HY, Jeong SH, Back JU et al. Hyarluronic acid hydrogels cross-linked by polyethlene glycol diglycidyl ether (PEGDE) for long-lasting dermal filler applications. 2016; 4.doi: 10.3389/conf.FBIOE.2016.01.01.809
- Philipp-Dormston WG, Hilton S et al. A prospective, open label, multicentre, observational, post market study of the use of 15mg/mL hyaluronic acid dermal filler in the lips. J Cosmet Dermatol. 2014;13(2):125-134. https://doi.org/10.1111/jocd.12085
- Sundaram H, Rohrich RJ, Liew S et al. Cohesivity of hyaluronic acid fillers: development of clinical implications of a novel assay, pilot validation with a five-point grading scale, and evaluation of six U.S Food and Drug Administration-approved fillers. Plast Reconstr Surg. 2015;136(4):678-86. https://doi.org/10.1097/PRS.0000000000001638
- Tran C, Carraux P, Micheels P et al. In vivo bio-integration of three hyaluronic acid filler in human skin: a histological study. Dermaltology. 2014; 228(1): 47-54. https://doi.org/10.1159/000354384
- Walker, K, Basehore BM, Zito PM, Hyaluronic Acid. Text Stat Pearls Publishing; 2018.

